Supplier Qualification Audits
Pre-Inspection Audits
Combination Audits
CMS/GAP Audits
Independent Audits-IGAP
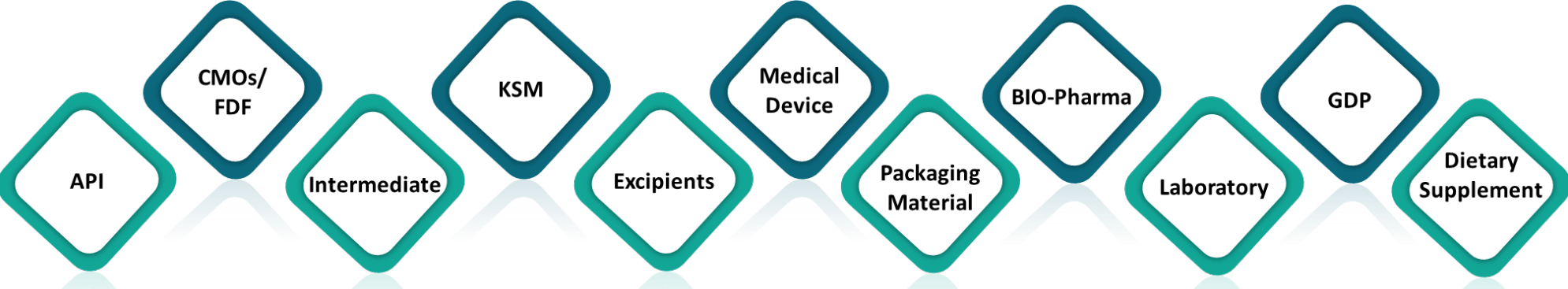
(API/CMO/Intermediate/MD.)
Supplier Qualification Audits
Pre-Inspection Audits
Combination Audits
CMS/GAP Audits
Independent Audits-IGAP
USFDA/ EU/ WHO/ PICs/ GMP Certification Quality System Design/ Development/ Implementation
Post Inspection /Remediation (483s / Warning Letters)
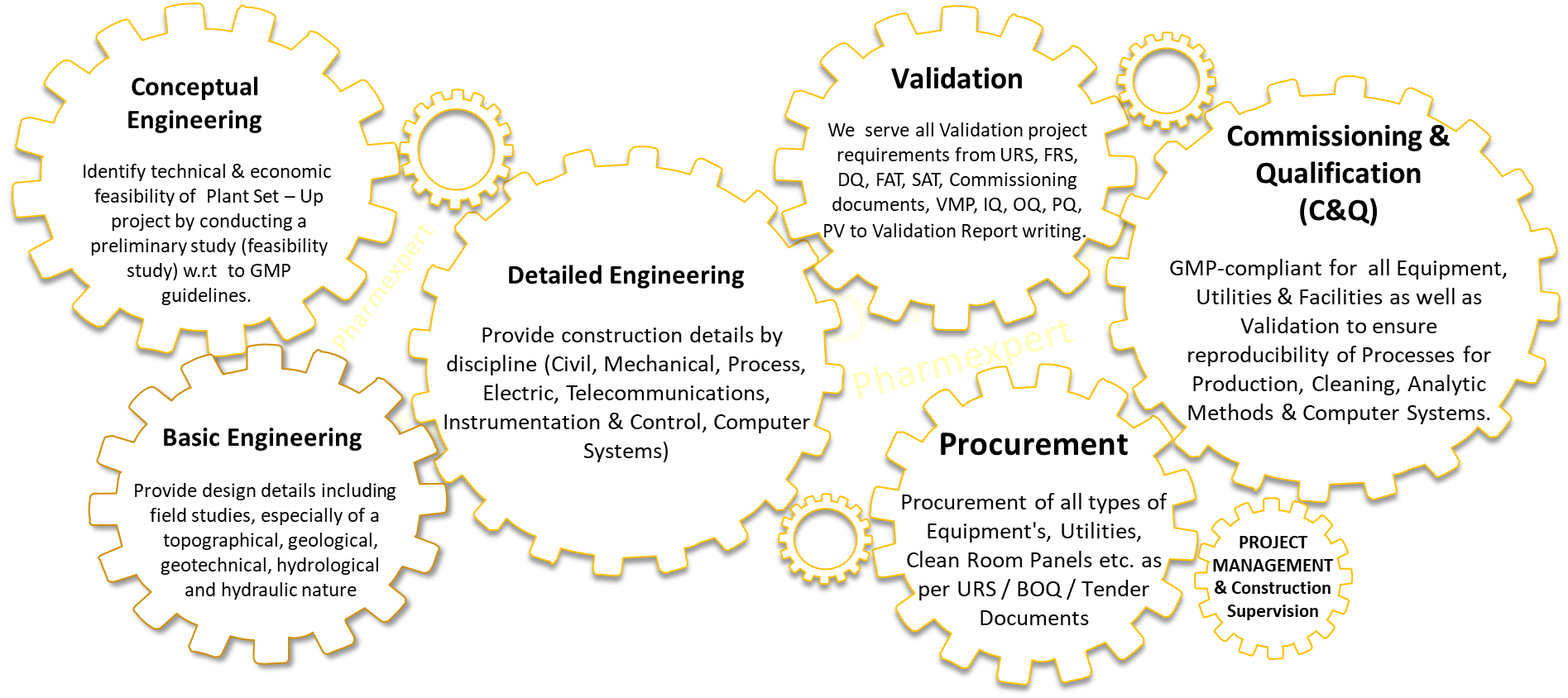
Conceptual, Basic, Detailed Engineering Project Management & Construction Supervision
Procurement Support
Commissioning & Qualification (C&Q) Validation
Pharma Plant Set-up
QP Services
Monitoring
Contract manufacturing
Training
Computerized System Validation

Wide range of Audit Services
SOP Driven Risk based approach
Our GMP experts have conducted various site audits including for API, Excipients, Key Starting Materials Intermediates, Formulations (OSD, Parenteral), Medical Device manufacturer as per USFDA, EMA, WHO, ISO compliance across the globe.
- 360 degree audit approach to evaluate the capabilities and quality systems of suppliers and out-sourcing partners
- To identify and resolve non-compliances in advance of a visiting regulatory agency
- And when on-site visits are not feasible, our flexible delivery model allows us to guarantee business continuity and the lowest possible impact on all auditing operations, we perform audits remotely via documentation reviews and SME discussions as per Pharmexpert methodology. And When onsite audit feasible, We plan for onsite audit.
- Review of all Systems for GMP Compliance & efficiency
- To investigate a process/Lab failure/OOS/OOT and determine the root cause and CAPA
- To evaluate the quality systems for sustainability
- To ensure systems are 21 CFR Part 11 compliant and have undergone sufficient validation
- To evaluate facility’s compliance as per regulatory standards set forth in 21 CFR Part 820, ISO 13485 and EU medical device (EMD)
- To ensure R&D and Analytical Laboratory compliance as per GLP standards & 21 CFR part 11 compliance
- We frequently carry out suppliers audits worldwide to ensure regulatory compliance.
- Joining our IGAP program/ Audit report library is free for suppliers/manufacture. Our experienced and qualified auditor will perform full site audit of supplier covering large number of molecules/products (if not already performed) with no charge to supplier and detailed audit report shall be prepared.
- The audit report shall be then available in our Audit report database which can be accessible to any of your clients. We will obtain permission of the supplier before sharing reports to the clients.
- When report needed on urgent basis to support the supply, regulatory submissions etc., When manufacturing site can’t accommodate the audit due to busy schedule, When travel restriction; Sponsor can get benefits by purchasing our IGAP reports from our report database, moreover our clients only spend a small fraction of the costs usually associated with conducting a supplier audit. Our Quality system ensure that our clients receive personalized reports with areas most relevant to their operations.
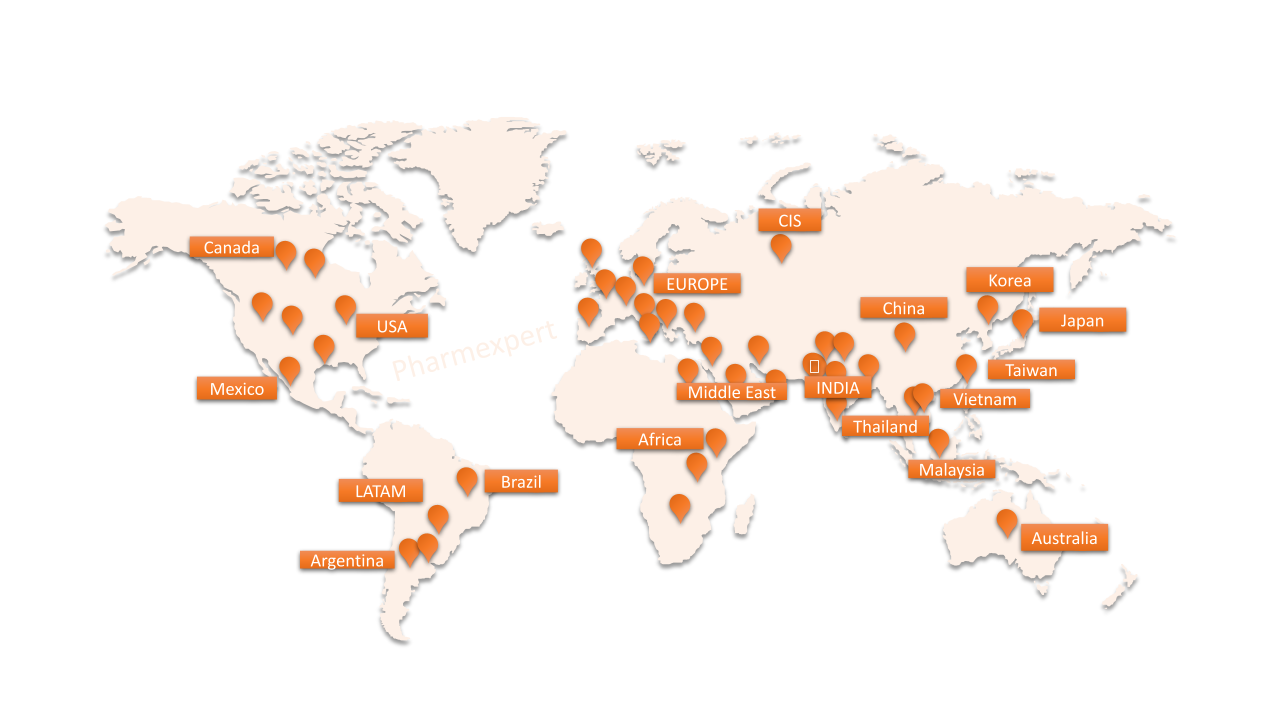
- Only one audit to be faced which will be valid for next 3 years. It will reduce your efforts and manpower involved hosting numbers of audits and implementing the ample no. of CAPAs generated through the multiple audit observations. Our cGMP Auditors qualified under Pharmexpert’s Methodology apart from having International certifications from APIC, IRCA-CQI, ECA and other and available locally across the globe including India, China, Japan, Korea, Canada, Europe, US, Taiwan, Malaysia, etc. We have completed more than 700+ Vendor/Supplier Qualification audits. Locally based consultants in wider geography so effective quality of work without language barrier and travel restrictions.
Our Audit reports are very detailed and comprehensive and accepted by all the regulatory authorities USFDA, EMA, PICs, WHO. The Audit report package includes Audit agenda, Audit Report, Auditors CV, CAPA Report, Audit Closure Letter, No conflict of interest statement by Auditor.
- Procuring ready audit report available from our Audit report database
- Sponsor can join Pharmexpert audit program –IGAP
USFDA/EU/ WHO/ PIC/s/ANVISA GMP Certification
We support site for successfully passing inspections and design post-inspections remediation plans to establish & upgrade GMP compliance and maintain approval for your product with Pharmexpert's tailored and cost-effective programs for Quality Management for Pharmaceuticals, Bio-Pharma and MD.




QMS set up
Manufacturing, Laboratory, Engineering
SOP development
Qualification for Equipment, Utility and Facility
Process, Analytical And Cleaning Validation support
Risk assessment for system, Facility, Process.
Training
Support in critical event management (complaints, deviations, sterility failures, recalls)
CAPA System Implementation
Sterility Assurance etc.

Pharmexpert, with diversified teams with and integrated knowledge in API, FDF, Bio-Pharma and more, can support clients with cost effective engineering solutions for Pharmaceutical, APIs, Biopharma and MD facilities to ensure that the related regulatory requirements set by Regulatory Authorities are met.
- We ensure that your plant site meets your business objectives while being fully compliant with the latest Good Manufacturing Practice regulations and standards.
- Extensive experience for complete setup of pharmaceutical manufacturing unit which includes IV fluids, Injectable, Tablet, Capsule, Liquid orals / externals, Powders, Bulk drugs, Ointment / Cream manufacturing plants.
- Handling of Green /Brown Field Projects.
- Product Release
- API / Formulation QP Audits & Certification
- Documents Evaluation
- Permanent / Short Term QP Cover
- Quality System Review
- Risk Management
- In-process monitoring of manufacturing operations: Include Dispensing to Packaging of the final product
- Help to find best quality CMOs worldwide. With a guarantee of GMP Compliance & Pharmexpert Reliability.
Leaner Centric Approach
From basic GxP to advanced specific trainings
- Our Experienced subject matter experts provide correct training on all GXP topics, Policies, Procedures, Documentation, Data Integrity and Customized topics to enhance operational excellence and serve as best tool to mitigate risks at cost effective manner.
Learn More...
System validation includes but not limited to
- ERP Systems/ DMS/ Network & PC based Systems Facility/ Utility Systems/ Barcode Systems / Warehouse Management Systems/ Building Automation Systems/ Computerized Maintenance Management Systems /PLC/DCS/SCADA/MES/EBRS/Automated Process Equipment
Learn More...